[ad_1]
A new solution from Penn Medicine scientists could reduce the time it takes to alter patients’ immune cells for infusion back into the human body to find and attack cancer. The mobile manufacturing course of action for this form of immunotherapy that was pioneered at Penn—CAR T mobile therapy—typically usually takes 9 to 14 days. In a pre-medical analyze revealed in Nature Biomedical Engineering, a group in the Perelman Faculty of Medication at the University of Pennsylvania abbreviated this procedure and created practical Motor vehicle T cells with improved anti-tumor potency in just 24 hours.
These success show the likely for a wide reduction in the time, supplies, and labor required to make Vehicle T cells, which could be primarily advantageous in patients with speedily progressive ailment and in useful resource-bad health care environments. The review was led by Middle for Mobile Immunotherapies scientists Michael C. Milone, MD, Ph.D., an affiliate professor of Pathology and Laboratory Medication and Saba Ghassemi, Ph.D., a investigation assistant professor of Pathology and Laboratory Drugs.
“Whilst standard production ways employed to produce Car T cells that consider many days to months keep on to perform for patients with ‘liquid’ cancers these kinds of as leukemia, there is even now a substantial need to have to lower the time and cost of producing these elaborate therapies” Milone said. “Making on our analysis from 2018 that reduced the regular production solution to a few times, and now to fewer than 24 hours, the production process described in this analyze is a testament to the opportunity to innovate and strengthen the generation of Auto T cell therapies for the benefit of additional clients.”
Vehicle T mobile remedy is a kind of immunotherapy utilized to fight cancer with a patient’s individual altered immune cells. T cells are taken from a patient’s blood and modified in the lab by introducing a gene for a receptor (named a chimeric antigen receptor or Car or truck). The Car or truck T cells are then infused back into a individual to uncover, bind to, and demolish cancer cells. On the other hand, when taken out as well extended from the entire body through the engineering course of action, T cells can shed their ability to replicate, which is central to their performance as a dwelling drug. Hence, the Penn investigate team sought to shorten the approach with out sacrificing the T mobile potency.
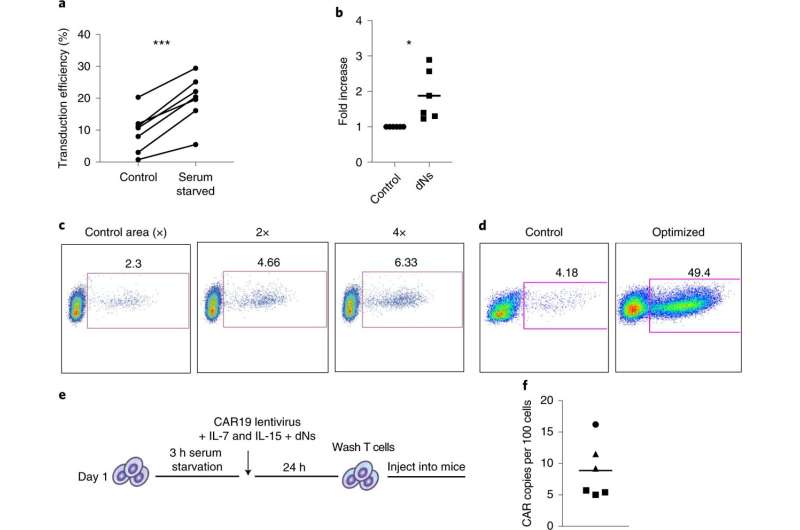
In animal designs, the scientists realized that the high-quality, rather than the quantity, of the Car or truck T cell item is an crucial determinant of their efficacy. Their experiment delivered evidence that a lesser number of high-quality Car T cells that are created without having in depth expansion exterior the body is outstanding to a better number of reduced-high-quality Automobile T cells that are thoroughly expanded prior to returning to the affected individual.
Regular producing techniques require T cells to be stimulated (or “activated”) in a way that induces the cells to replicate and increase in variety. A important to the Penn researchers’ manufacturing technique is the lentiviral vector that provides the Auto gene to the T cells. Lentiviral vectors, which are derived from the human immunodeficiency virus (HIV), are equipped to transfer genes like the Motor vehicle to cells without the need of the require for this first “activation” phase, but the efficiency of this procedure was reduced. Using engineering approaches that designed in aspect upon information of how HIV the natural way infects T cells, the Penn scientists developed a way to conquer this prerequisite for T cell activation and supply genes specifically to non-activated T cells freshly isolated from the blood. This had a dual profit of expediting the over-all producing procedure whilst also sustaining T mobile potency. Clients are not becoming contaminated with HIV via this approach.
The approach of engineering T cells is costly and time-intensive, given that the cure must be manufactured for each and every unique affected person. The team hopes that reducing manufacturing time could make the therapy much more price tag-efficient and obtainable to extra people.
“This ground breaking tactic is remarkable in that it may well be equipped to help people who may in any other case not be in a position to profit from Automobile T mobile treatment this kind of as these with speedily progressing cancer thanks to important time at present need to have to produce these therapies,” Ghassemi stated. “Effective reprogramming of T cells with a Vehicle in as very little as 24 hours in a more simplified production approach with no T mobile activation or in depth tradition outside the house the physique also presents the likelihood of growing in which and when these therapies are made. Not only could possibly it strengthen the generation potential of centralized producing amenities, but if straightforward and constant sufficient, it may possibly be achievable to produce these therapies domestically near the affected person, which could be tantamount to addressing the numerous logistical worries that impede supply of this successful remedy particularly in source-lousy environments.”
This research is a catalyst for more medical research to examine how the engineered Vehicle T cells, by this shortened approach, function in individuals with precise cancers.
Penn researchers led investigation, improvement and clinical trials of this pioneering Vehicle T remedy in collaboration with Novartis and Children’s Medical center of Philadelphia. In 2017, the experimental therapy now regarded as Kymriah, became the to start with Vehicle T mobile accredited by the U.S. Foodstuff and Drug Administration (Food and drug administration), for the remedy of pediatric and young adult patients with acute lymphoblastic leukemia (ALL). The therapy was also accredited for certain styles of lymphoma in 2018.
Saba Ghassemi et al, Fast producing of non-activated powerful Vehicle T cells, Mother nature Biomedical Engineering (2022). DOI: 10.1038/s41551-021-00842-6
Citation:
Scientists shorten manufacturing time for Motor vehicle T mobile therapy (2022, March 29)
retrieved 7 April 2022
from https://medicalxpress.com/information/2022-03-shorten-motor vehicle-cell-therapy.html
This doc is issue to copyright. Aside from any honest dealing for the reason of private study or study, no
portion may well be reproduced without the composed permission. The articles is delivered for information functions only.
[ad_2]
Resource connection







